This is an archival copy of the Visualization Group's web page 1998 to 2017. For current information, please vist our group's new web page.
3D Microtubule Reconstruction
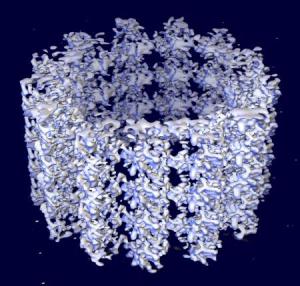
|
Ken Downing's group has obtained a 3-D reconstruction of intact microtubules, using cryo-electron microscopy and image processing, at a resolution of about 8 Å, sufficient to resolve much of the secondary structure. Rather than use helical image processing methods, they have treated the microtubule images as strings of single particles. The structures of the alpha and beta monomers are sufficiently similar that, at this resolution, we can ignore the difference between them. The resulting map is thus an average of the alpha and beta tubulin structures. The level of detail in the map allows docking of the tubulin crystal structure with very strong constraints, providing several important insights not previously available through docking tubulin into lower resolution maps.
This work provides an improved picture of the interactions between adjacent protofilaments that are responsible for microtubule stability and also suggests that some structural features are different in microtubules from those in the zinc sheets in which the tubulin structure was determined. The N-terminal loop is even more important than the H3 helix in forming the inter-protofilament interface with the M-loop. The small H6 domain is less involved in inter-protofilament contacts than in the crystals, and may be flexible enough to allow regulatory ligands such as Taxol to diffuse through the microtubule wall and bind to the inside surface. A cluster of residues that differ among beta tubulin isotypes is located at the major inter-protofilament interaction site, which may relate to differences in dynamic properties and drug sensitivities observed for the different isotypes.
Microtubules play fundamental roles throughout the life of eukaryotic cells. These roles often involve dynamic activities of the microtubules themselves and interactions with other proteins that modulate microtubule stability. The dynamic behavior of microtubules has been the subject of great interest for several decades, but only recently has the structure of tubulin allowed us to begin to understand the molecular basis of the dynamics. However, in order to advance our knowledge of the regulation of the microtubule cytoskeleton, it is essential that we develop a better understanding of the interactions among the tubulin subunits within a microtubule. The structure of the intact microtubule, which we report on here, reveals new inter-protein interactions and conformational changes associated with the formation of microtubules.
Advances in the methodology of single particle reconstructions have made it feasible to consider this approach to obtaining resolution of at least 10 Å with microtubules, treating each image as a string of single particles. This approach allows one to take account of distortions within a single microtubule that limit resolution with helical processing. Using a combination of single particles and crystallographic methods, we have obtained a microtubule reconstruction at 8 Å resolution. Most of the alpha helices in the dimer structure can be identified in this map, providing a very strong constraint in placement of the atomic structure. This docking gives an improved picture of the inter-protofilament interactions and suggests some conformational differences in tubulin between microtubules and Zn-sheets.
This image was created by Ken Downing, with assistance from the LBL Visualization Group, and on the high capacity visualization hardware located at the LBL Visualization Laboratory.